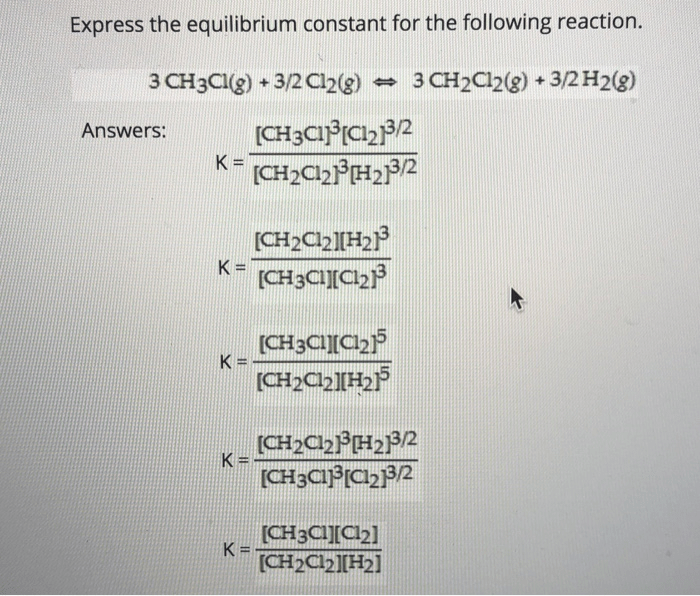
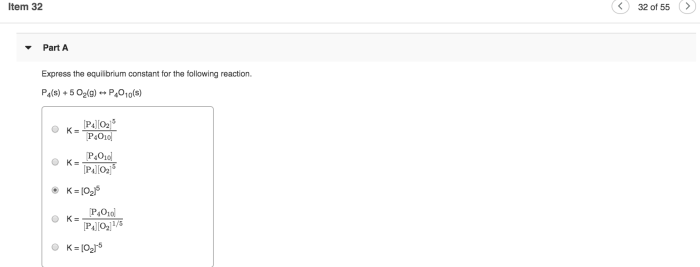
Express the equilibrium constant for the following reaction – Expressing the equilibrium constant for a given reaction is a crucial aspect of understanding chemical reactions and their behavior. It allows us to quantify the extent to which a reaction proceeds and predict its direction under various conditions.
This guide provides a comprehensive overview of the concept of equilibrium constant, its formula, and its significance in chemical reactions. We will explore the factors that affect the equilibrium constant and discuss its applications in predicting reaction outcomes and optimizing reaction conditions.
Understanding the Equilibrium Constant: Express The Equilibrium Constant For The Following Reaction
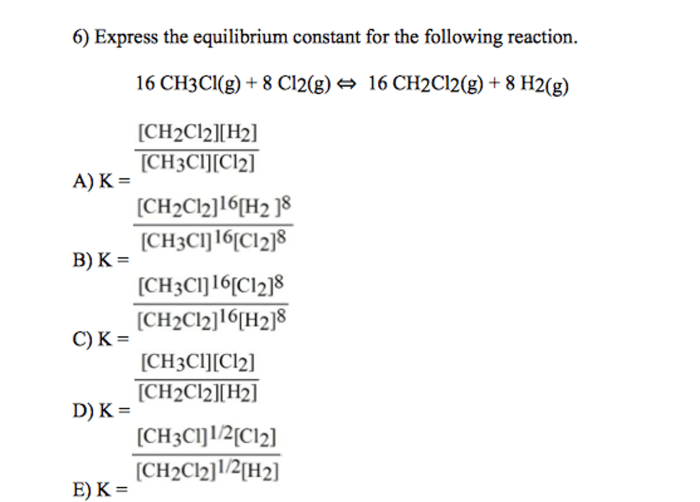
An equilibrium constant (Keq) is a quantitative measure of the extent to which a chemical reaction proceeds towards completion. It represents the ratio of the concentrations of the products to the concentrations of the reactants at equilibrium, a state where the forward and reverse reactions occur at equal rates.
The equilibrium constant is a crucial parameter in chemical reactions, providing insights into the spontaneity and direction of the reaction. It allows chemists to predict the extent to which a reaction will proceed, estimate the concentrations of reactants and products at equilibrium, and optimize reaction conditions.
For example, consider the reaction:
A + B ⇌ C + D
The equilibrium constant for this reaction is:
Keq = [C][D]/[A][B]
Where [A], [B], [C], and [D] represent the concentrations of the respective species at equilibrium.
Expressing the Equilibrium Constant
The general formula for expressing the equilibrium constant is:
Keq = (Πa_products)/(Πa_reactants)
Where:
- Πa_products is the product of the activities of the products, each raised to its stoichiometric coefficient in the balanced chemical equation.
- Πa_reactants is the product of the activities of the reactants, each raised to its stoichiometric coefficient in the balanced chemical equation.
Activities are effective concentrations that account for non-idealities in the solution. For dilute solutions, activities can be approximated by concentrations.
The units of the equilibrium constant depend on the stoichiometry of the reaction. For example, the equilibrium constant for a reaction involving gases is expressed in units of pressure, while for reactions involving solutes, it is expressed in units of concentration.
Factors Affecting the Equilibrium Constant
Several factors can shift the equilibrium of a reaction, thereby affecting the equilibrium constant.
- Temperature:Increasing temperature generally favors the endothermic reaction (absorbs heat), while decreasing temperature favors the exothermic reaction (releases heat).
- Concentration:Increasing the concentration of reactants shifts the equilibrium towards products, while increasing the concentration of products shifts the equilibrium towards reactants.
- Pressure:For reactions involving gases, increasing pressure shifts the equilibrium towards the side with fewer moles of gas, according to Le Chatelier’s principle.
Understanding these factors is essential for controlling and optimizing chemical reactions.
Applications of the Equilibrium Constant, Express the equilibrium constant for the following reaction
The equilibrium constant has numerous applications in chemistry:
- Predicting Reaction Direction:By comparing the value of Keq to 1, we can predict the direction of a reaction. If Keq > 1, the reaction proceeds towards products, while if Keq< 1, the reaction proceeds towards reactants.
- Calculating Reaction Yields:The equilibrium constant can be used to calculate the maximum yield of products that can be obtained under specific conditions.
- Optimizing Reaction Conditions:By manipulating factors that affect the equilibrium constant, such as temperature and concentration, chemists can optimize reaction conditions to maximize product yield and minimize side reactions.
The equilibrium constant is a powerful tool that enables chemists to understand, predict, and control chemical reactions.
Detailed FAQs
What is the equilibrium constant?
The equilibrium constant is a numerical value that expresses the relative amounts of reactants and products at equilibrium. It indicates the extent to which a reaction proceeds and the direction in which it will shift to reach equilibrium.
How is the equilibrium constant expressed?
The equilibrium constant is typically expressed as a ratio of the concentrations of products to reactants, each raised to its stoichiometric coefficient. For a general reaction aA + bB ⇌ cC + dD, the equilibrium constant (K) is given by K = [C]^c[D]^d / [A]^a[B]^b.
What are the units of the equilibrium constant?
The units of the equilibrium constant depend on the reaction and the units used for the concentrations. For reactions involving gases, the equilibrium constant is often expressed in terms of partial pressures. For reactions in solution, the equilibrium constant is typically expressed in terms of molar concentrations.