Draw the predominant form of histidine at ph 0 – Drawing the predominant form of histidine at pH 0 unveils the intricacies of protonation and deprotonation, providing insights into the behavior of this versatile amino acid in biological systems.
Histidine, with its unique imidazole ring, exhibits pH-dependent protonation states, influencing its structure, function, and interactions within the cellular environment.
Predominant Form of Histidine at pH 0

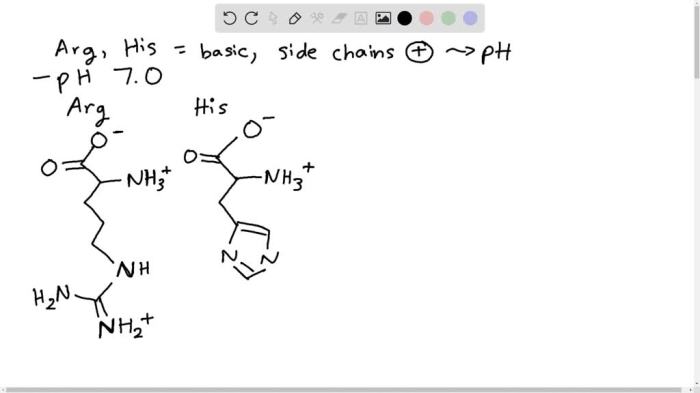
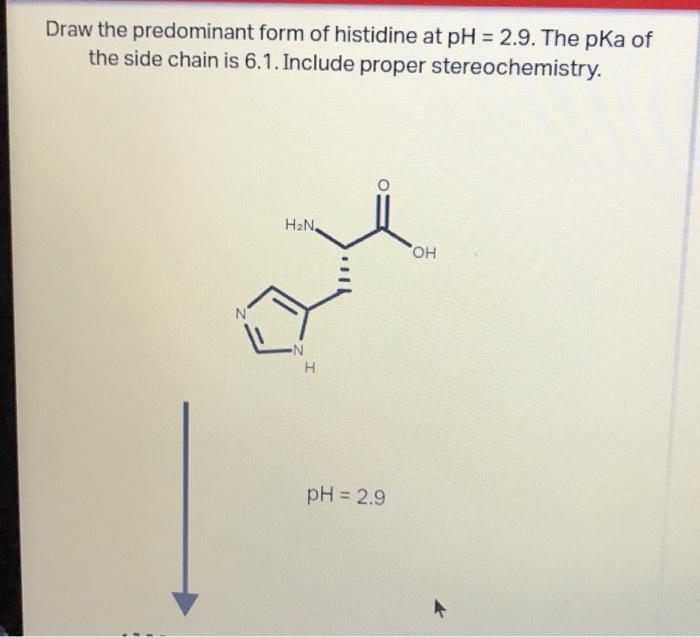
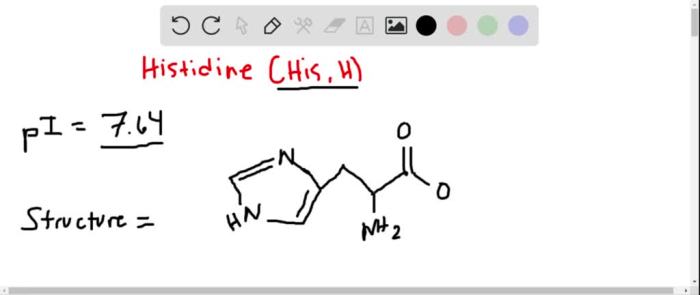
Histidine is an amino acid with a unique chemical structure that allows it to exist in several protonated and deprotonated forms. At pH 0, the predominant form of histidine is the fully protonated form, with a positive charge on the imidazole ring and a positive charge on the amino group.
Protonation is the process of adding a hydrogen ion (H+) to a molecule, while deprotonation is the process of removing a hydrogen ion. The protonation and deprotonation of histidine are governed by the pH of the solution. At low pH, the concentration of hydrogen ions is high, which favors protonation.
At high pH, the concentration of hydrogen ions is low, which favors deprotonation.
The chemical structure of histidine at pH 0 is shown below:

Factors Affecting Histidine Protonation
The predominant form of histidine is influenced by several factors, including pH, temperature, and ionic strength.
pH
pH is the most important factor affecting histidine protonation. As the pH increases, the concentration of hydrogen ions decreases, which favors deprotonation. Conversely, as the pH decreases, the concentration of hydrogen ions increases, which favors protonation.
Temperature
Temperature has a small effect on histidine protonation. As the temperature increases, the protonation constant decreases, which favors deprotonation. This is because the increased thermal energy disrupts the hydrogen bonds between histidine and hydrogen ions.
Ionic strength
Ionic strength has a small effect on histidine protonation. As the ionic strength increases, the protonation constant decreases, which favors deprotonation. This is because the increased ionic strength reduces the activity of hydrogen ions.
Applications of Histidine Protonation: Draw The Predominant Form Of Histidine At Ph 0
Histidine protonation has several important applications in biological systems and biotechnology.
Buffering
Histidine is an effective buffer in biological systems. This is because it can exist in several protonated and deprotonated forms, which allows it to absorb or release hydrogen ions as needed to maintain a constant pH.
Enzyme catalysis
Histidine is involved in the catalytic mechanism of many enzymes. This is because the protonation and deprotonation of histidine can alter the activity of the enzyme’s active site.
Biotechnology
Histidine protonation is used in several biotechnological applications. For example, it is used to purify proteins by ion-exchange chromatography and to design biosensors for detecting pH changes.
Experimental Techniques for Studying Histidine Protonation
Several experimental techniques can be used to study histidine protonation.
Spectroscopic techniques, Draw the predominant form of histidine at ph 0
Spectroscopic techniques, such as nuclear magnetic resonance (NMR) and ultraviolet-visible (UV-Vis) spectroscopy, can be used to study the protonation state of histidine. These techniques can provide information about the chemical environment of the histidine residue and the number of protons that are bound to it.
Potentiometric titrations
Potentiometric titrations can be used to determine the protonation constants of histidine. This is done by titrating a solution of histidine with a strong acid or base and measuring the pH of the solution. The protonation constants can then be calculated from the titration data.
Computational methods
Computational methods, such as molecular dynamics simulations, can be used to model histidine protonation. These methods can provide detailed information about the protonation state of histidine and the factors that affect it.
FAQ Overview
What factors influence the predominant form of histidine?
pH, temperature, and ionic strength are key factors that affect histidine protonation.
How is histidine protonation studied experimentally?
Spectroscopic techniques (NMR, UV-Vis), potentiometric titrations, and computational methods are commonly used to investigate histidine protonation.
What are the applications of histidine protonation?
Histidine protonation plays crucial roles in biological buffering, enzyme catalysis, and potential biotechnology applications.