Embark on a scientific voyage to unravel the captivating enigma: is KBr polar or nonpolar? Join us as we delve into the molecular realm, exploring the intricate interplay of atomic arrangements and their profound impact on a compound’s behavior.
Prepare to be captivated by a journey that illuminates the fascinating world of chemical polarity, where the dance of electrons unveils the secrets of matter’s interactions.
Definition of Polarity
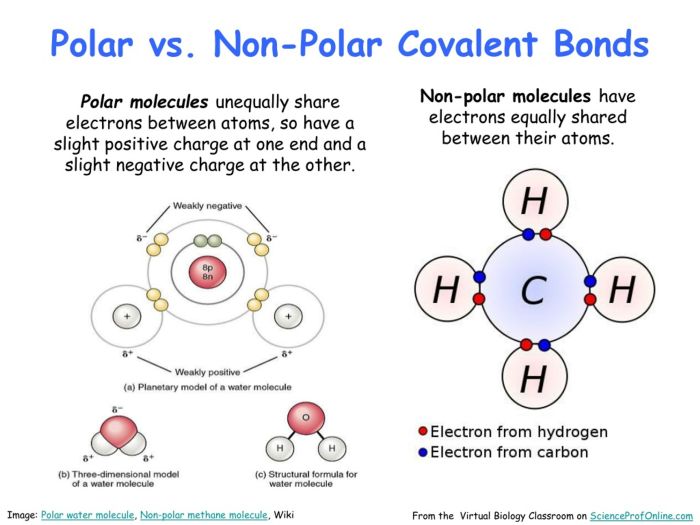
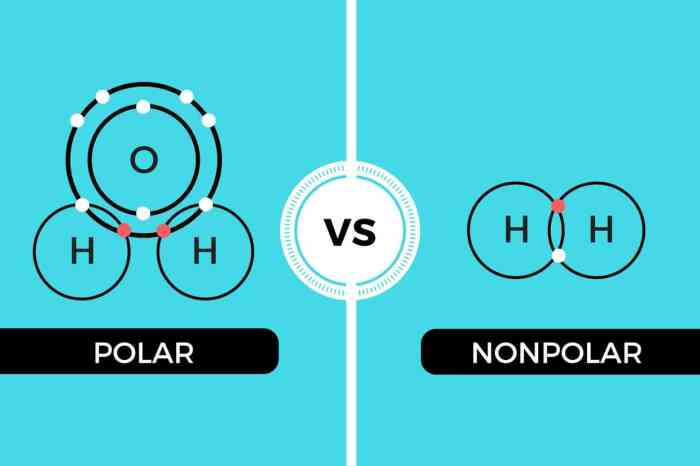
Polarity refers to the uneven distribution of electrical charge within a molecule. A molecule is considered polar if it has a positive end and a negative end. This charge separation arises due to differences in electronegativity between the atoms that make up the molecule.
Electronegativity is the ability of an atom to attract electrons towards itself. When atoms with different electronegativities bond, the more electronegative atom will pull the shared electrons closer to itself, creating a partial negative charge on that atom and a partial positive charge on the less electronegative atom.
Polar vs Nonpolar Molecules
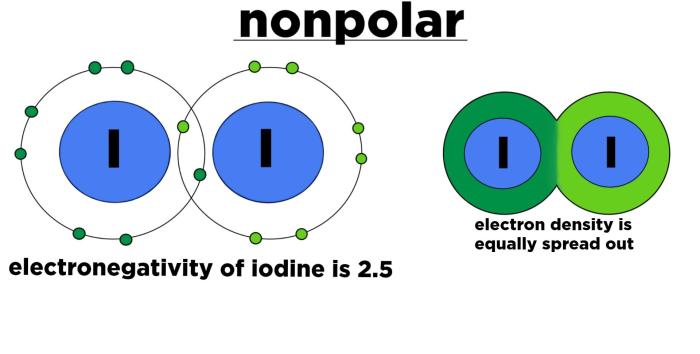
Based on polarity, molecules can be classified as either polar or nonpolar. Polar molecules have a permanent dipole moment, meaning they have a separation of positive and negative charges even in the absence of an external electric field. Nonpolar molecules, on the other hand, have no permanent dipole moment and their electrons are evenly distributed.
- Polar molecules: These molecules have a net positive or negative charge due to the uneven distribution of electrons. Examples include water (H2O), ammonia (NH3), and hydrogen chloride (HCl).
- Nonpolar molecules: These molecules have no net charge and their electrons are evenly distributed. Examples include methane (CH4), carbon dioxide (CO2), and benzene (C6H6).
Molecular Structure of KBr: Is Kbr Polar Or Nonpolar
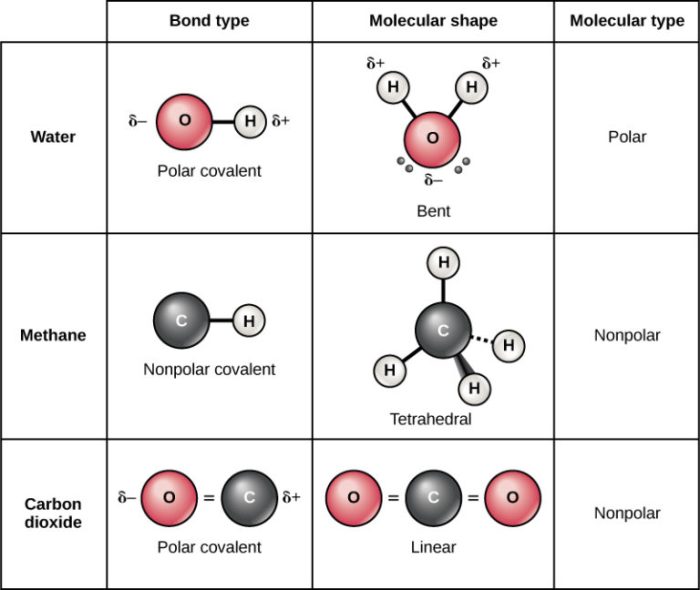
Potassium bromide (KBr) is an ionic compound composed of potassium (K+) and bromide (Br-) ions. The molecular structure of KBr can be described as a cubic crystal lattice, where each potassium ion is surrounded by eight bromide ions, and vice versa.
Is KBr polar or nonpolar? That’s a question for the chemistry buffs. But if you’re looking for a bit of literary wit, check out this pun in Romeo and Juliet . It’s a classic example of wordplay that’s sure to make you smile.
And while we’re on the subject of puns, let’s get back to our original question: is KBr polar or nonpolar?
This arrangement forms a repeating pattern throughout the crystal.
Polarity of KBr
The polarity of a molecule is determined by the distribution of its electrons. In KBr, the potassium ion has a positive charge, while the bromide ion has a negative charge. The difference in electronegativity between potassium and bromine causes the electrons to be drawn towards the bromine ion, resulting in a polar covalent bond between the two ions.
However, since KBr is an ionic compound, the electrons are completely transferred from potassium to bromine, forming a fully ionic bond. As a result, KBr is considered a nonpolar compound.
Properties of KBr
KBr is an ionic compound formed by the electrostatic attraction between potassium ions (K+) and bromide ions (Br-). The polarity of a compound refers to the uneven distribution of electrons within its molecules. Polar compounds have a separation of charges, with one end of the molecule being slightly positive and the other end being slightly negative.
Nonpolar compounds, on the other hand, have a symmetrical distribution of electrons, resulting in no separation of charges.
Polarity and Properties, Is kbr polar or nonpolar
The polarity of KBr affects its physical and chemical properties. Polar compounds tend to be soluble in polar solvents, while nonpolar compounds are soluble in nonpolar solvents. This is because polar solvents have a separation of charges that can interact with the polar molecules, while nonpolar solvents do not.
Additionally, polar compounds tend to have higher boiling points and melting points than nonpolar compounds. This is because the electrostatic interactions between the polar molecules require more energy to overcome in order to change their state.
Table: Properties of Polar and Nonpolar Compounds
| Property | Polar Compounds | Nonpolar Compounds ||—|—|—|| Solubility | Soluble in polar solvents | Soluble in nonpolar solvents || Boiling Point | Higher | Lower || Melting Point | Higher | Lower |
Applications of KBr
KBr’s ionic character and its ability to transmit infrared radiation make it useful in various applications.
KBr finds applications in the following areas:
Infrared Spectroscopy
KBr is commonly used in infrared (IR) spectroscopy. It is employed to prepare sample pellets for IR analysis. KBr is transparent to IR radiation and does not absorb IR light in the mid-IR region, making it an ideal matrix for IR sample preparation.
To prepare a KBr pellet, a small amount of the sample is mixed with finely ground KBr powder and pressed into a transparent disk. The IR spectrum of the sample can then be obtained by shining IR radiation through the KBr pellet.
Optics
KBr is used in optics due to its high refractive index and transparency in the visible and near-infrared regions. It is employed in the production of lenses, prisms, and windows for optical instruments.
KBr’s high refractive index allows it to bend light more effectively, making it suitable for use in lenses and prisms. Its transparency in the visible and near-infrared regions enables it to transmit light without significant absorption or scattering.
Medicine
KBr has been used in medicine as a sedative and anticonvulsant. It was once commonly prescribed to treat epilepsy, but its use has declined due to the development of more effective and safer medications.
KBr works by depressing the central nervous system, reducing neuronal excitability. However, its use is limited by its potential for side effects, including drowsiness, nausea, and skin rashes.
FAQ
Is KBr a polar or nonpolar compound?
KBr is a polar compound due to the difference in electronegativity between potassium and bromine.
What factors determine the polarity of a compound?
The polarity of a compound depends on the electronegativity difference between its constituent atoms and the molecular geometry.
How does polarity affect the properties of a compound?
Polarity influences a compound’s solubility, boiling point, and melting point, among other properties.